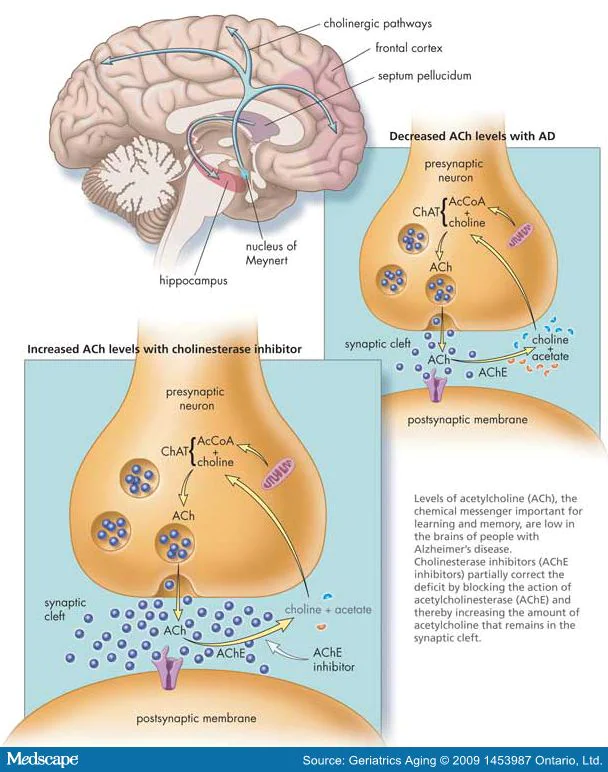
Recent studies suggest a different approach to elaborating therapies for these neurodegenerative disorders. In general, one of the typical pathological hallmarks of Parkinson’s (PD) and Alzheimer’s disease (AD) is the misfolding and accumulation of specific proteins like the α-synuclein and the β-amyloid, which, in high levels, are detrimental to the brain cells.[4] Even though most of the research effort is used to prevent the accumulation of these proteins, there is still no cure for neurodegeneration. However, several studies have pointed out that amyloids (β-amyloid) and α-synuclein oligomers are more linked to the symptoms of both disorders.[1-3] Oligomers are toxic and aggregate early before forming α-synuclein clumps and β-amyloid plaques—equally.[3] These oligomers are formed from the self-assembly of a few molecules of amyloid proteins. There are two oligomers: (1) the soluble oligomers and (2) the membrane-associated oligomers.[1]
Interestingly, only the membrane-associated oligomers are noxious when interacting with the plasma membrane.[3] Some articles refer to AD as a membrane disorder.[3] The membrane-oligomer interaction is generally arbitrated by molecules called gangliosides.[2] Gangliosides are assembled micro-domains of the membrane that form lipid rafts. [2,5] Gangliosides are used as connection sites by α-synuclein and β-amyloid proteins.[3] For a membrane-oligomer connection, first, they need to bind to gangliosides.[2] Once the oligomers bind the gangliosides, they form “amyloid pores”.[2,3] Amyloid pores are slight channels in which unregulated Ca+2 ions enter the cell, generating a calcium imbalance in brain cells.[3] In normal conditions, calcium is crucial in plasticity, signaling between neurons, synaptic transmission, and transport.[3,6]
On the other hand, the unregulated entrance of Ca+2 ions inside the cells caused an increase in toxicity and led to apoptosis (cell death).[4] In addition, high levels of Ca+2 ions stimulate the activation of pathways for the overproduction of other proteins like the tau protein, another pathological hallmark of AD. [6] Oxidative stress, synaptic decline, and plasticity have also been observed.[3] Therefore, finding molecules able to bind and block the gangliosides can help prevent amyloid-related oligomers from attaching and forming the amyloid pores, thus helping reverse or prevent the development of AD and PD.[1,3,5]
AmyP53 is a peptide (KEGVLYVGHHTK) used in recent investigations to develop new treatments for AD and PD.[3] AmyP53 was created with the necessary binding properties of the oligomers to bind the gangliosides, thus avoiding the amyloid oligomers to bind and form the amyloid pores (see Fig. 1).[3] Once the AmyP53 prevents the amyloid pore formation, the Ca+2 ions maintain its average level. AmyP53 also prevents neuroinflammation, synaptic loss, neuronal death, tau misfolding, and mitochondrial dysfunction, which can all cause the development of neurodegenerative disorders, especially AD and PD.[1-4] To develop this peptide, the investigators identified recognition sites in the ganglioside structures and then tested different molecules that share the molecular characteristics for binding. [1-3] The Amyp53 peptide can be administered successfully through intravenous and intranasal administration.[3] However, the recommended method is the intranasal administration (nasal spray) (see Fig.2). [1,3] This peptide can be used as a treatment for both AD and PD because of the mechanism used. Recent studies have also shown that AmyP53 has excellent stability in temperatures up to 45 ◦C for months without signs of degradation.[3] In addition, the AmyP53 can cross the blood-brain barrier through both the intranasal and intravenous administration, where higher amounts of the peptide are found in the brain than in the blood.[1]